- All Exams Instant Download
What would happen to the pressure of the gas if the temperature inside the can increases?
Gases are one of three common states of matter and are affected by changes in temperature, volume, and pressure. When the pressure of a gas in a closed container becomes too high, it can cause the container to burst. The graph below illustrates the relationship between pressure and temperature (K) for ordinary gases.
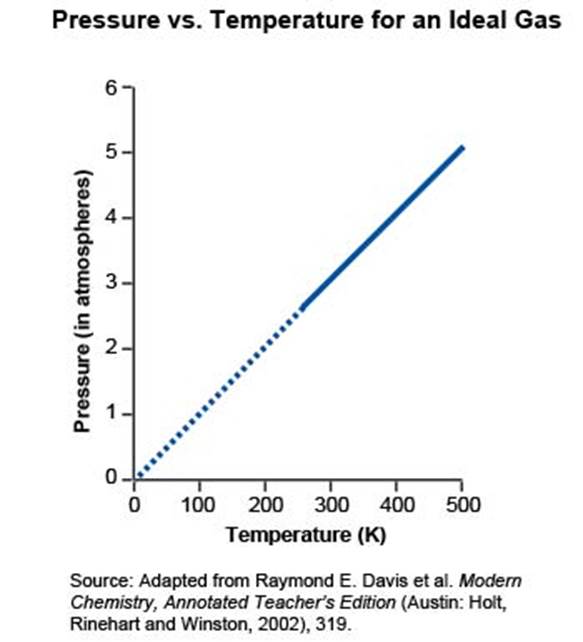
Aerosol cans contain a pressurized gas that is used to propel, or spray, the contents out of the can. Many aerosol cans have labels that warn the users against incinerating (burning) them because of the danger of explosion.
What would happen to the pressure of the gas if the temperature inside the can increases?
A . vary
B . increase
C . decrease
D . remain the same
E . increase, and then decrease
Answer: B
Latest GED Science Dumps Valid Version with 300 Q&As
Latest And Valid Q&A | Instant Download | Once Fail, Full Refund
Subscribe
Login
0 Comments
Inline Feedbacks
View all comments

