You are conducting an audit at a single-site organisation seeking certification to ISO 9001 for the first time. The organisation manufactures cosmetics for major retailers and the name of the retailer supplied appears on the product packaging
DRAG DROP
You are conducting an audit at a single-site organisation seeking certification to ISO 9001 for the first time. The organisation manufactures cosmetics for major retailers and the name of the retailer supplied appears on the product packaging. Sales turnover has increased significantly over the past five years
You are interviewing the new Product Development Manager. You note that a software application called SWIFT is used to help control the product development process.
You have gathered audit evidence as outlined in the table. Match the ISO 9001 clause 8.3 extracts to the audit evidence.

To complete the table click on the blank section you want to complete so it is highlighted in red and then click on the ISO 9001 Clause 8.3 extracts listed below. Alternatively, drag and drop each clause to the audit evidence that applies.
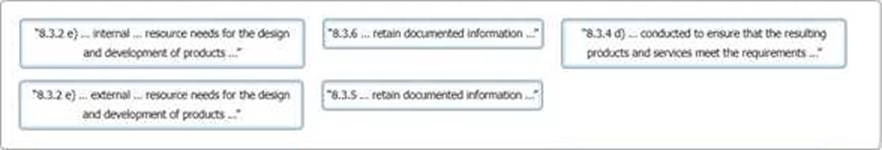
Answer: 
Explanation:
The table below shows the possible matching of the ISO 9001 Clause 8.3 extract to the audit
evidence.
Table
Audit evidence
Half of all new products launched in the past 12 months were late. The NPD Manager explains he has not got enough people on his team to cope with the demand for new products.
The NPD Manager explains many changes are made to cosmetic formulations during product development owing to retailer feedback. Only when confirmed by the retailer is the agreed formulation documented on SWIFT.
The NPD Manager explains that the customer confirms their approval to
ISO 9001 Clause 8.3 extract
“8.3.2 e) … internal … resource needs the design and development of prod …”
“8.3.5 … retain documented informat …”
“8.3.6 … retain documented informat
proceed with a new formulation by email. These emails are kept on SWIFT.
The NPD Manager shows you evidence of consumer trials that are carried out for some new products prior to full-scale launch.
The NPD Manager explains that an approved external laboratory is used to perform shelf-life stability trials on some formulations during product development.
…”
“8.3.4 d) … conducted to ensure that resulting products and services meet requirements …”
“8.3.2 e) … external … resource need the design and development of prod …”
Latest ISO-9001 Lead Auditor Dumps Valid Version with 226 Q&As
Latest And Valid Q&A | Instant Download | Once Fail, Full Refund

